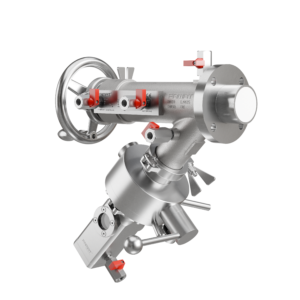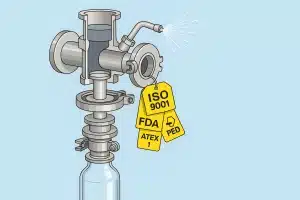
In highly regulated industries like pharmaceuticals, biotechnology, and fine chemicals, to be GMP compliant isn’t optional, it’s foundational. Every piece of equipment that comes into contact with the product must meet strict hygiene, traceability, and safety standards. Sampling valves are no exception.
Although often seen as a minor component, sampling valves play a critical role in ensuring product quality and batch consistency. A poorly designed valve can introduce contamination, create dead zones where product accumulates, or fail under pressure, leading to costly production downtime or even product recalls.
At FAMAT Sampling, our sampling solutions are engineered from the ground up to exceed GMP expectations. From design materials to cleanability and certification, every element is optimized to meet the strictest global standards.
What Does GMP Compliance Mean for a Sampling Valve?
To meet GMP guidelines, a sampling valve must go far beyond basic functionality. GMP-compliant design prioritizes cleanliness, traceability, and repeatable performance, especially under high-pressure or sterile conditions. Here are the core requirements:
- Dead-space-free design: Valves must prevent residue build-up. FAMAT’s patented piston remains flush with the vessel wall in the closed position, eliminating potential contamination points.
- Hygienic surface finishes: Internal roughness must be Ra ≤ 0.8 µm or lower, ensuring product doesn’t cling to surfaces. Polished or electropolished finishes are often required.
- FDA-compliant materials: All contact parts must be made of certified materials like 316L stainless steel, Hastelloy®, and FDA-approved seals (e.g., EPDM, FFKM, PTFE).
- Easy cleaning and validation: GMP valves must be compatible with CIP/SIP systems and allow for full disassembly where necessary, something FAMAT’s Tri-Clamp “easy clean” models do exceptionally well.
- Traceability and documentation: Full material traceability and qualification documents (e.g., EN10204 3.1 certificates) must be available for audits and regulatory inspections.
These design principles are embedded in every FAMAT valve, ensuring consistent performance in sterile, high-risk environments.
Core GMP Design Features in FAMAT Sampling Valves
At FAMAT, GMP compliance is not an add-on, it’s embedded into the engineering of every sampling valve. Our patented Expanding Piston Technology (EPT®) is a perfect example. This system creates a bubble-tight seal without relying on fragile O-rings, which can degrade, generate particles, and compromise cleanliness. The result: a dead-zone-free, contamination-resistant valve that ensures process integrity batch after batch.

Each valve is designed for maximum cleanability. The piston sits flush with the vessel wall when closed, eliminating corners where residue might collect. For operations that require frequent batch changes, our Tri-Clamp “easy clean” versions allow quick disassembly and full access to all product-contact surfaces, without special tools.
From low surface roughness (Ra ≤ 0.8 µm, with optional Ra ≤ 0.4 µm) to FDA-approved elastomers and fully customizable configurations, FAMAT valves are built to handle the strictest hygienic and operational requirements.
Certifications That Matter (and Why They Matter)
Regulatory compliance starts with certified equipment. FAMAT valves are backed by a full suite of internationally recognized certifications that give quality and safety teams complete confidence:
- ISO 9001:2015 – Our manufacturing process is certified for quality management and traceability
- PED 2014/68/EU – All pressure equipment complies with the European Pressure Equipment Directive
- FDA Compliance – All elastomers in contact with the product are FDA-approved
- ATEX 2014/34/EU – Equipment is certified for use in explosive or hazardous environments
- Optional: ISO 15848-1 for low fugitive emissions, API 607 for fire safety, and more
These certifications aren’t just badges, they’re essential for companies undergoing GMP audits by agencies like the EMA or FDA. With FAMAT, clients can trust that compliance is already built into the valve’s DNA.
How FAMAT Customizes Sampling Valves for GMP Applications
No two production lines are the same, especially in industries like pharmaceuticals and biotech, where every process has unique containment, cleaning, and integration needs. That’s why FAMAT offers fully customizable GMP-compliant sampling valves tailored to each client’s setup.
From standard DN15, DN25, and DN50 models to special nose lengths, cleaning ports, or actuator-ready versions, each FAMAT valve can be configured for exact operational requirements. Outlet types like GL45 thread, Tri-Clamp, or Bayonet are selected based on existing equipment or sterility protocols. Seals are available in a wide range of FDA-approved materials, including PTFE, EPDM, and FFKM, to suit temperature, chemical compatibility, or cleanroom constraints.
Conclusion
In regulated environments, small design choices can have big consequences. A sampling valve that isn’t truly GMP-compliant can become a weak point in an otherwise sterile process, leading to contamination, regulatory issues, or product loss.
FAMAT’s expertise is 100% focused on sampling, and it shows in every solution we deliver. From dead-zone-free design to certified materials, cleanability, and custom integration, we make GMP compliance simple, reliable, and scalable.