Highly Potent Active Pharmaceutical Ingredients (HPAPIs) are a category of compounds used in medications that deliver powerful therapeutic effects at extremely low doses. Because of their potency, even minimal exposure can pose serious health risks to operators, making safety a top priority in their manufacturing and handling.
HPAPIs are increasingly used in modern medicine, especially in oncology, hormone therapies, and targeted treatments for chronic conditions. Their ability to work in small quantities helps reduce side effects for patients, but it also demands advanced containment technologies to protect manufacturing personnel and maintain product integrity.
With regulatory agencies such as the EMA and OSHA enforcing strict occupational exposure limits (OELs), the pharmaceutical industry must adopt highly controlled systems to ensure safe and compliant processing of HPAPIs.
The Hidden Risk in HPAPI Manufacturing: Sampling
While much attention is given to large-scale containment systems and isolators, one of the most overlooked, yet critical, risks in HPAPI production is sampling.
Sampling often requires breaking containment to collect material for quality control, batch release, or process monitoring. If not handled correctly, this small act can result in significant operator exposure, product contamination, and regulatory non-compliance.
Traditional sampling setups are prone to leaks, require complex engineering, or depend on bulky isolators. These limitations increase costs, slow down validation, and compromise safety in confined environments. That’s why having a dedicated, high-containment sampling system is essential for any company handling HPAPIs.
Why OEB4/OEB5 Containment Matters
In HPAPI manufacturing, safety is measured by Occupational Exposure Bands (OEBs), which classify compounds based on their toxicity and the level of containment required to handle them safely. The most potent HPAPIs fall into OEB4 and OEB5, meaning even nanogram-level exposure can harm operators.
Quick OEB Overview:
| OEB Level | Typical Exposure Limit (µg/m³) | Containment Required |
| OEB1 | >1,000 | Basic PPE |
| OEB3 | 10–100 | Contained systems |
| OEB5 | <1 | Isolators / advanced containment |
Many manufacturers still rely on gloveboxes, full isolators, or cleanrooms to meet OEB5 requirements, but these setups are expensive, hard to scale, and difficult to maintain.
That’s where FAMAT’s compact sampling system stands out: it delivers OEB5-level protection without the need for large-scale infrastructure, enabling safer handling in small and mid-scale operations.
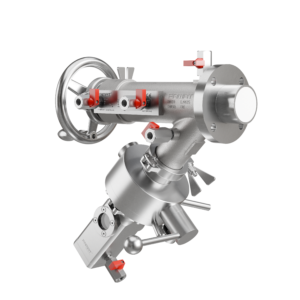
FAMAT’s High-Containment Sampling Solution
FAMAT’s advanced sampling system is engineered for safe, aseptic sampling of highly potent compounds, without compromising performance or operator safety.
Equipped with an integrated split butterfly valve, this system enables precise, contamination-free sample collection while maintaining a closed containment loop. Designed to seamlessly integrate with reactors, dryers, mixers, and other confined processing equipment, the solution helps manufacturers avoid the cost and complexity of isolators.
🔧 Key Technical Highlights:
- ✅ Up to OEB5-level containment
- ✅ Minimal operator exposure and product contamination
- ✅ Compact, modular design
- ✅ Easy-to-clean, CIP/SIP-compatible components
- ✅ Reduces cleaning validation workload
Whether you’re working in small-batch manufacturing or scaling up HPAPI production, FAMAT’s solution provides the performance of an isolator, in a footprint the size of a valve module.
Use Cases & Integration Examples
FAMAT’s high-containment sampling system is built for real-world versatility. Whether you’re operating in pilot plants, GMP production environments, or CDMOs managing small-batch oncology drugs, this solution adapts seamlessly to your setup.
💡 Common Integration Points:
- Reactors: Inline sampling during active batch processes
- Dryers: Safe sample collection without opening containment
- Mixers: Monitoring blend uniformity in confined systems
- R&D Labs: Testing small volumes with full containment
Thanks to its compact footprint and modular design, the system is ideal for facilities with limited space but high safety demands. It’s especially suited for manufacturers transitioning from traditional APIs to HPAPIs, where retrofitting isolators may be cost-prohibitive.
Cost, Scalability & Validation Advantages
Unlike traditional isolator systems, which often involve significant upfront investment and long lead times, FAMAT’s containment solution offers a leaner, faster alternative without compromising safety or compliance. The system eliminates the need for costly gloveboxes or large-scale isolators, significantly reducing capital expenditure. Its pre-validated modules also streamline the qualification process, allowing teams to reduce setup and documentation efforts. Cleaning and maintenance are simplified thanks to CIP/SIP compatibility, minimizing downtime and labor. Most importantly, the system is designed with scalability in mind, manufacturers can expand or modify their setup without needing a complete redesign. This flexibility makes it an ideal choice not only for large-scale production but also for agile, growing operations that prioritize both safety and return on investment.
Comparing Containment Technologies
When evaluating high-containment solutions for HPAPI sampling, it’s important to understand how different technologies stack up. Traditional isolators have been the industry norm, but they come with significant drawbacks in terms of space, cost, and flexibility. FAMAT’s modular sampling system offers a modern alternative, without the bulk, delays, or operational burden.
| Feature | Traditional Isolator | FAMAT Sampling System |
| OEB Level | Up to OEB5 | Up to OEB5 |
| Installation Footprint | Large | Compact |
| Capital Investment | High | Low |
| Cleaning & Validation | Complex | Streamlined (CIP/SIP-ready) |
| Scalability | Limited | Modular and expandable |
| Integration with Equipment | Requires retrofitting | Plug-and-play into reactors, dryers, mixers |
For facilities aiming to modernize without overhauling their infrastructure, FAMAT delivers isolator-level protection in a smarter, leaner package.
Conclusion
The rise of HPAPIs in modern pharmaceuticals demands smarter, safer, and more adaptable containment solutions, especially during high-risk operations like sampling. Traditional systems like isolators, while effective, are often bulky, expensive, and difficult to scale.
FAMAT’s high-containment sampling system offers a new standard. By combining OEB5-level protection with a compact, modular design, it enables pharmaceutical manufacturers and CDMOs to ensure operator safety, maintain product integrity, and simplify validation, all without the cost or complexity of outdated infrastructure.
If you’re looking to upgrade your HPAPI containment strategy or reduce risks in your current setup, we’re here to help.