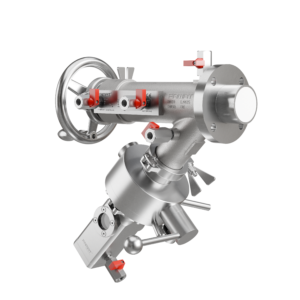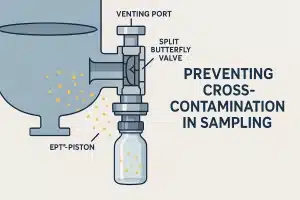
In pharmaceutical and chemical manufacturing, cross-contamination is one of the most dangerous and costly process failures. It can compromise product quality, trigger batch recalls, and pose serious health risks, especially in HPAPI environments or multiproduct facilities. Yet during sampling, one of the most routine operations, contamination often goes unnoticed until it’s too late.
Sampling involves temporarily interrupting containment to collect product, which, if not properly managed, becomes a prime entry point for airborne particles, product residue, or cleaning chemical backflow. The risk is compounded by factors like dead zones in valve bodies, poor cleanability, or inadequate bottle handling.
Common Causes of Cross-Contamination in Sampling
Most contamination during sampling isn’t due to human error, it’s built into the system. Poor valve design, aging seals, or incompatible cleaning protocols can introduce contaminants that affect product purity or expose operators to hazardous substances.
The most common causes include:
- Dead zones inside the valve where product accumulates and degrades over time
- Seals and O-rings that wear down, shed particles, or create microleaks
- Difficult-to-clean interfaces between the valve and the sampling bottle
- Open bottle handling, exposing product to airborne particles or glove contact
- Lack of purge capability, preventing pressure equalization and drawing contaminants inward
- Reused bottles or hoses, especially without proper sterilization between batches
Even if procedures are followed perfectly, design flaws like these make cross-contamination likely. The solution lies in smarter engineering, not just better cleaning.
Engineering Controls to Minimize Contamination Risk
Preventing cross-contamination starts with the design of the sampling system itself. Equipment must be engineered not only for performance, but for hygiene, repeatability, and complete isolation during operation. At FAMAT, this is where we focus most of our innovation.
One of the most effective design features is our Expanding Piston Technology (EPT®), which creates a seal directly between the piston and valve body, without O-rings. This eliminates the risk of elastomer wear, dust shedding, and microgaps where product can accumulate. When the piston is in the closed position, it’s completely flush with the vessel wall, ensuring zero dead space.
Surface finish is another critical factor. FAMAT valves are machined to an internal roughness of Ra ≤ 0.8 µm, with options down to Ra ≤ 0.4 µm upon request. This smooth surface resists residue buildup and allows for more effective cleaning.
For operations requiring sterility or high containment, our valves can be equipped with split butterfly valves that isolate both the sampling bottle and valve outlet. Combined with venting ports and purge options, this enables clean, controlled pressure equalization before opening the system, dramatically reducing exposure and contamination risk.
Cleaning, Validation & Documentation Protocols
Even the most hygienically designed valve can become a contamination risk without proper cleaning and validation procedures. That’s why CIP (Clean-in-Place) and SIP (Sterilize-in-Place) compatibility is non-negotiable for any sampling system used in GMP environments.
FAMAT valves are engineered to allow full access for cleaning media through integrated cleaning ports. Whether cleaning is automated or manual, the flush piston design and dead-zone-free architecture ensure that no residue is left behind. For more intensive needs, models like the Tri-Clamp “easy clean” series enable rapid disassembly without special tools, so inner components can be visually inspected, cleaned, or autoclaved between batches.
From a compliance perspective, documentation is just as important. Our valves come with:
- Material traceability certificates (EN10204 3.1)
- FDA approval declarations for wetted seals and elastomers
- Validation support for cleaning protocols
This level of traceability helps engineering and quality teams align with GMP, FDA, and EMA guidelines during audits or regulatory reviews.
Bottle & Interface Handling: Small Detail, Big Impact
Cross-contamination doesn’t just happen inside the valve, it often occurs at the connection point between the valve and the sampling bottle. Poorly secured bottles, exposed threads, or reused containers can compromise an otherwise sterile process.
FAMAT addresses this through secure, sealed bottle interfaces available in multiple formats: Bayonet, Tri-Clamp, and GL45 thread, depending on process needs. All bottle contact materials are FDA-approved and available in borosilicate glass with stainless steel protection or fully enclosed metallic designs for high-containment scenarios.
Additional safety features include:
- Venting valves for pressure equalization before disconnecting
- Purge connections to eliminate trapped air or particulates
- Mechanical locking systems that prevent bottle removal unless the valve is fully closed
These engineering details are essential for avoiding contamination caused by handling or transfer, and for protecting both your product and your people.
High-Risk Use Cases: When Containment Matters Most
Cross-contamination during sampling is a universal risk, but in certain manufacturing environments, its consequences can be far more severe. This is especially true in the production of highly potent APIs (HPAPIs), where even minimal exposure can endanger operators and compromise product safety. In multiproduct facilities, residual traces from a previous batch can jeopardize the integrity of the next, making proper isolation essential. Cleanroom and aseptic processing zones also demand high standards, as any breach in sterility can result in significant product loss and regulatory consequences.
Facilities operating in explosive or hazardous environments governed by ATEX regulations face an added layer of complexity. Here, every component must meet strict material and performance standards to ensure safety and compliance. In all of these cases, the choice of sampling equipment can make or break the integrity of the process. FAMAT valves, with their robust sealing systems and full compliance with OEB5 containment levels, are designed specifically for these high-stakes scenarios.
How FAMAT Solves Cross-Contamination Challenges
FAMAT’s commitment to precision sampling goes beyond standard valve manufacturing. Our engineering process begins with a simple goal: eliminate contamination risk at the source. This is achieved through design innovations like our Expanding Piston Technology (EPT®), which replaces fragile O-ring seals with a self-expanding piston that creates a tight, particle-free closure. The piston sits flush with the vessel wall when closed, eliminating dead space and buildup zones that are often the hidden cause of contamination.
Cleaning and maintenance are streamlined thanks to CIP/SIP-compatible designs, while Tri-Clamp “easy clean” options allow fast disassembly for full visual inspection. Sampling bottles are secured using reinforced interfaces such as Bayonet, GL45, or Tri-Clamp connections, with options for venting and purging to maintain pressure control and product isolation. These systems are further reinforced by optional locking mechanisms, ensuring that the sample cannot be removed until the system is fully sealed.
FAMAT’s design philosophy centers on flexibility and reliability. We work closely with engineering teams, integrators, and equipment manufacturers to deliver valves that fit seamlessly into complex process environments. Whether for a compact R&D line or a high-containment commercial facility, our solutions are tailored to ensure long-term safety, compliance, and performance.
Conclusion
Cross-contamination is one of the most overlooked threats in pharmaceutical and chemical sampling, yet it’s entirely preventable when the right engineering decisions are made. Poor sealing, hard-to-clean designs, or unsecure bottle handling can quickly become weak points in an otherwise controlled system.
FAMAT eliminates these risks by embedding compliance, hygiene, and safety directly into the valve’s architecture. With decades of expertise, proven performance in critical applications, and a strict focus on GMP-ready designs, we help manufacturers operate with confidence at every stage of production.