In pharmaceutical manufacturing, contamination isn’t just a possibility, it’s a liability. Every exposed surface, every moving part, and every sampling interaction represents a potential breach. That’s why sampling valves are under increasing scrutiny from auditors, especially in facilities handling HPAPIs, biologics, or other high-potency drug products.
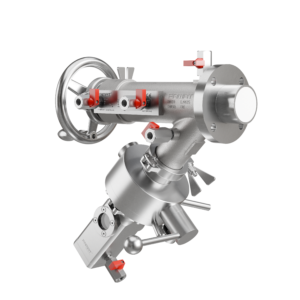
At FAMAT Sampling, we’ve developed Expanding Piston Technology (EPT®) to directly address these risks. Unlike traditional valves, our patented design eliminates common failure points, such as dead zones and O-ring wear, with a containment-focused approach built for GMP compliance, repeatable validation, and cleanroom integration.
Why Contamination Risk Is a Major Issue in Pharmaceutical Sampling
Contamination can compromise not only product quality but also operator safety and regulatory standing. Sampling is one of the few stages in pharmaceutical manufacturing where the product must interface directly with equipment and human operators, creating a critical risk point.
Whether dealing with powders, granules, suspensions, or liquids, microbial or particulate contamination at the sample point can invalidate entire batches. This is especially problematic in the case of non-sterile pharmaceutical products, where ensuring microbiological quality requires tight control at every stage of the sampling procedure.
Sampling errors or design flaws have led to costly recalls, rejected batches, and even regulatory sanctions. It’s no longer acceptable to treat sampling valves as minor components. For inspectors and manufacturers alike, they’re now part of the core GMP risk landscape.
Where Traditional Sampling Valves Fall Short
Most conventional valves rely on static seals, O-rings, or threaded components that introduce micro-gaps into the product path. These gaps, often overlooked, become dead zones where residual product, moisture, or microorganisms can accumulate.
Over time, these micro-environments are difficult to clean and prone to microbial contamination, especially in non-sterile drug products. CIP/SIP processes may not fully reach or flush them, and visual inspection often fails to detect hidden buildup. The result? An invisible risk that grows with each batch cycle.
What Makes EPT® a Contamination-Resistant Design
EPT® eliminates the classic weak points by using a self-expanding piston that achieves a flush, crevice-free seal every time. There are no exposed elastomers in the product path, no complex geometries, and no areas that trap residue. Once sampling is complete, the piston retracts smoothly, ensuring zero dead volume is left behind.
This seamless design improves cleanability and ensures each sampling cycle starts with a truly sterile environment, especially when paired with validated CIP/SIP protocols. It also helps manufacturers meet stringent GMP and ISO 2859 expectations for cleanliness and consistency.
Reducing Cross-Contamination in Multiproduct Environments
In facilities handling multiple APIs, excipients, or dosage forms, cross-contamination is a daily concern. Traditional sampling systems may carry microscopic residues from one batch to the next, especially if original samples aren’t properly isolated or if the valve design hinders full cleaning validation.
EPT® dramatically lowers this risk by minimizing internal surface complexity and making full cleaning verification possible. Its geometry supports composite sampling without material crossover, ensuring batch integrity and easier documentation, a key point during audits.
Validation and Regulatory Confidence Built In
One of the core advantages of EPT® lies in how easily it integrates into process validation. Because the piston mechanism creates a repeatable seal with every use, it supports reliable performance testing, traceable cleaning cycles, and consistent sampling outcomes. This predictability aligns with quality assurance expectations and satisfies GMP inspectors looking for proof of contamination control.
Additionally, EPT® valves are compatible with standard analytical testing, allowing operators to verify cleanliness post-cleaning through swab or rinse tests. That means you’re not just assuming your equipment is clean, you can prove it with data.
Conclusion: Engineering Out Contamination Risk
EPT® isn’t just an upgrade to conventional valves, it’s a rethink of how contamination risks are addressed at the sampling point, one of the most sensitive stages in any pharmaceutical process. With its flush closure, seal-free product path, and validated cleanability, it provides a straightforward, auditable solution to a longstanding challenge.
Whether you’re managing non-sterile pharmaceutical products, handling potent APIs, or preparing for your next regulatory audit, EPT® simplifies compliance and strengthens confidence in your sampling procedure, batch after batch.