In high-containment environments, especially those handling HPAPIs, biologics, or cytotoxic compounds, sampling systems are no longer just a technical detail. They’re a central focus during audits. Inspectors know that if contamination is going to happen, it often happens here.
Unlike sealed vessels or automated lines, sampling involves direct interaction between equipment, product, and operator. That makes it a high-risk point for cross-contamination, operator exposure, and regulatory non-compliance. During audits, inspectors go straight to the valve: Is there a dead zone? Can it be cleaned? Are the seals intact? Is there proper documentation?
A sampling system that doesn’t check these boxes can raise immediate red flags, even if everything else in your process is GMP-compliant. The good news is that these risks are avoidable, but only if the system is designed from the start with containment and validation in mind.
The Regulatory Context: What Auditors Expect from Containment Systems
GMP inspectors don’t just want to know that your sampling system works, they want to know that it works safely, consistently, and with proof.
Across global frameworks like EU GMP Annex 1, FDA cGMP, and ICH Q9/Q10, there’s a growing emphasis on risk-based design, cleanability, and contamination control. With the rise of high-potency products, regulators now expect documented compliance with Occupational Exposure Limits (OELs) and Occupational Exposure Bands (OEBs), especially levels 4 and 5. This means your sampling valve must do more than function; it must actively prevent contamination and operator exposure, with design features that support CIP/SIP, validation, and cleanroom integration.
Auditors are also increasingly asking to see the full technical file, certificates for materials, surface roughness specs, validation test results, and integration diagrams. Any design flaw or missing documentation here can delay approvals or trigger corrective action.
FAMAT sampling systems are engineered to meet these expectations head-on. In the next section, we’ll break down exactly what inspectors look for, and where standard valves often fall short.
What Inspectors Typically Evaluate in Sampling Systems
Dead Zones and Cleanability
Inspectors immediately check for internal spaces where product can accumulate. Any dead volume is a red flag, as it’s nearly impossible to clean reliably. FAMAT valves use flush pistons to eliminate this risk completely.
CIP/SIP Compatibility
Sampling systems must integrate seamlessly into cleaning cycles. Auditors look for built-in cleaning ports, validated spray coverage, and the ability to sterilize without full disassembly, unless tool-free access is available.
Seal Integrity and Wear
Gaskets and O-rings can degrade over time. Auditors want to see proof that seals don’t shed particles and that the valve can maintain integrity across cycles. FAMAT’s expanding piston technology removes O-rings entirely from the product path.
Documentation & Traceability
Inspectors will request material certificates, surface finish specs, and design pressure ratings. If those aren’t documented, or worse, if the system isn’t testable, you’re at risk.
Must-Have Design Features to Pass Inspection
To pass inspection, a high-containment sampling valve must do more than function, it needs to be engineered for compliance. Flush pistons that eliminate dead space, smooth internal surfaces (Ra ≤ 0.8 µm or better), and tool-free access for cleaning are no longer optional; they’re baseline expectations.
Inspectors also look at the integrity of seals and connections. FAMAT’s expanding piston design eliminates traditional O-rings in the product path, reducing wear and contamination risk. Secure bottle interfaces with vent and purge options, and high-containment split butterfly valves, help maintain sterility and isolation from the environment during sampling and transfer.
What Makes a System Truly Validatable
Inspectors don’t just evaluate how a system is built, they want to know how reliably it performs over time. A validatable sampling system must support repeatable results across batches, consistent cleaning outcomes, and full traceability of materials. This includes documented performance under pressure, exposure, and sterilization cycles.
What sets a system apart is its ability to integrate into the plant’s validation protocols. That means compatibility with existing CIP/SIP procedures, access to technical files, and availability of FAT (Factory Acceptance Test) documentation. A good system isn’t just compliant on paper, it makes audits easier because everything is traceable, testable, and built to meet the same standards as the rest of your cleanroom equipment.
How FAMAT Systems Are Built to Pass
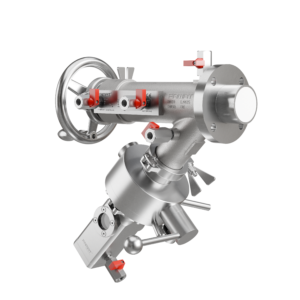
FAMAT sampling valves are engineered from the ground up with inspections in mind. Our patented Expanding Piston Technology (EPT®) eliminates contamination risks tied to traditional seals and provides a flush, dead-space-free closure. Every unit is available with documentation for material compliance, surface finish, and validation compatibility.
We offer modular designs that integrate seamlessly into your existing setup, whether you’re working with isolators, reactors, dryers, or mixers. From OEB5-ready valves with split butterfly modules to compact, easy-clean systems for multiproduct lines, FAMAT valves are not just built to perform, they’re built to pass.
Conclusion
Inspections don’t start with paperwork, they start with your equipment. Sampling systems are one of the first places auditors will look, and one of the most common places contamination risk is exposed.
FAMAT valves are built to meet, and exceed, regulatory expectations. If you’re preparing for an audit or upgrading your containment system, we’re ready to help you get it right.
High-Containment Sampling for HPAPIs
Looking to optimize your HPAPI containment strategy? Contact us today.


